Sevil Hakimi
Tabriz University of medical science, Iran
Title: Randomized controlled trial of abdominal binders for postoperative pain, distress, and blood loss after cesarean delivery
Biography
Biography: Sevil Hakimi
Abstract
Objective: To assess the effect of using abdominal binders on pain, distress, and postpartum hemorrhage after cesarean delivery.
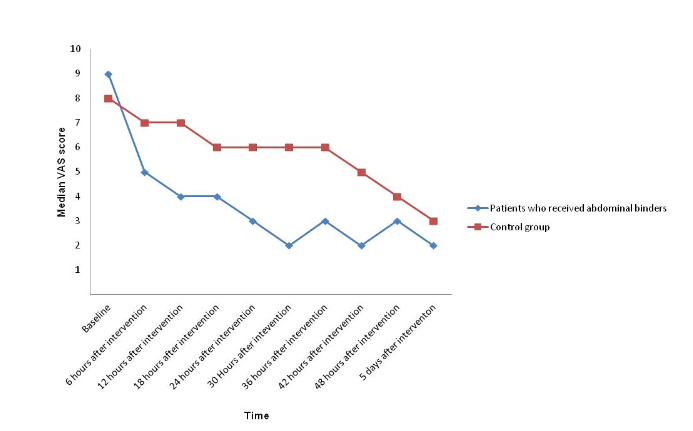
Methods: The present prospective randomized controlled trial enrolled patients undergoing non-emergency cesarean deliveries in Golestan Province, Iran. Patients were randomized in a 1:1 ratio by blocks of four or six to a control group or to use an abdominal binder after delivery; all patients received routine care. The primary outcomes were visual analog scale-assessed pain, symptom distress scale (SDS)-assessed distress and hemoglobin and hematocrit levels. Participants and researchers were masked to treatment assignments until after cesarean delivery and data analysis was unmasked; intention-to-treat analyses were performed.
Results: There were 89 patients enrolled in each group, with no differences in baseline pain scores, SDS scores and hemoglobin and hematocrit levels between the groups (all P>0.05). Pain and SDS scores were lower in the binder group at all post-baseline time points compared with the control group (all P<0.001). Hemoglobin and hematocrit levels were higher among patients who received binders 36 hours after baseline (both P<0.001). There was one patient who experienced hemorrhage in the binders group and one patient requested removal of their binder.
Conclusions: Patients who received abdominal binders reported less pain, lower SDS scores and higher hemoglobin and hematocrit levels following cesarean delivery.